Lixumistat (IM156) in Oncology
Lixumistat (IM156) Affects Tumor Metabolism
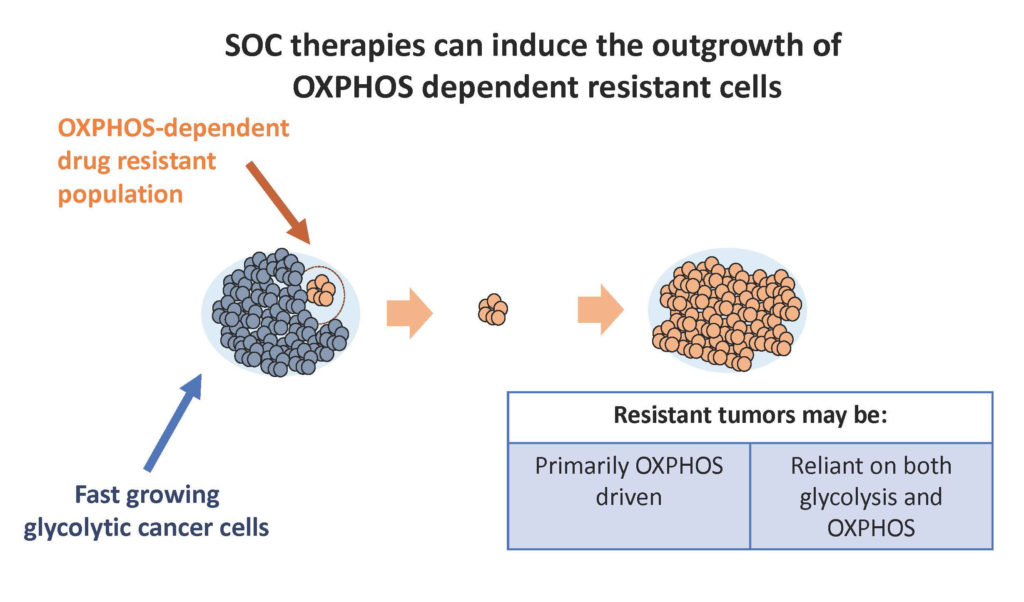
The development of resistance to anti-cancer treatments is a challenge that occurs frequently in cancer patients and results in limited duration of response, and this loss of efficacy occurs with chemotherapies, targeted therapies, and limits immunotherapies. Recent scientific/medical advances suggest that resistance is often associated with a metabolic profile that is defined as having increased OxPhos activity. This observation is consistent with stressed cells, like tumors cells responding to anti-cancer agents, requiring the increased energy and biosynthetic substrates that would be provided with increased mitochondrial OxPHhos activity. Additionally, elevated OxPhos activity results in a hypoxic tumor microenvironment caused by increased cellular oxygen consumption, and such hypoxia has been shown to limit the effectiveness of immunotherapies. Tumor cells that are resistant to current therapies due to dependence on elevated mitochondrial OxPhos activity are expected to be highly sensitive to metabolic inhibitors of OxPhos activity. Preclinical studies with OxPhos inhibitors continue to provide scientific rationale for the identification and targeting OxPhos-sensitive tumors and translation of the preclinical results to the clinic. ImmunoMet’s near-term focus is the identification of subpopulations of cancer patients whose tumors are dependent on OxPhos activity and the clinical demonstration of OxPhos inhibitors as novel anti-cancer therapeutics.
Lixumistat (IM156) Oncology Program Addresses Intrinsic & Acquired Resistance
Lixumistat (IM156) is an orally administered small molecule biguanide. Lixumistat (IM156) is a particularly promising PC1 inhibitor that has the potential to treat drug resistant tumors, whose disease is driven by OxPhos activity. Lixumistat (IM156) has demonstrated robust in vitro activity and in vivo efficacy in selected cancers and cancer subpopulations, including pancreatic ductal adenocarcinoma (PDAC), glioblastoma multiforme (GBM), gastric, lymphoma, and lung cancers. A Phase 1 dose escalation study to evaluate the safety and tolerability of lixumistat (IM156) in cancer patients with advanced solid tumors has been successfully completed. No dose limiting toxicities occurred in this study and a recommended Phase 2 dose was determined based on tolerability. Extended stable disease (up to approximately one year) was demonstrated in this unselected patient population. A recently completed Phase 1 dose escalation study confirmed and extended the pharmacokinetic and tolerability characterization of lixumistat (IM156) in healthy subjects and demonstrated target engagement at tolerable doses/exposures. Lixumistat (IM156) is the first Protein Complex 1 Inhibitor to demonstrate target engagement at tolerable doses and define a recommended Phase 2 dose.
Lixumistat (IM156): The Promise of a Safe and More Effective Therapy
At ImmunoMet Therapeutics, we are aggressively advancing a novel OxPhos inhibitor, lixumistat (IM156), as an anti-cancer therapeutic for those patients whose tumors are dependent on OxPhos activity.
